October 17, 2023
National Cancer Center Japan
Chiba University
Kavli Institute for the Physics and Mathematics of the Universe (WPI-Kavli IPMU)
Kyoto College of Medical Science
A team of researchers has developed a new method that suppresses the distribution of drugs to healthy tissues, but also to rapidly removes the drugs once distributed in the body, which could improve the accuracy of imaging diagnosis of difficult cancers, reduce toxicity to healthy tissues, and furthermore improve the effectiveness of treatment, reports a recent study published in the Journal of Controlled Release.
Radiopharmaceuticals have been attracting attention as the next generation of cancer imaging and cancer treatment drugs. Until now, most research has focused on the developing the technology to deliver drugs to cancer lesions, but it has been impossible to avoid distributing drugs to healthy tissues.
A team of researchers, led by National Cancer Center Exploratory Oncology Research & Clinical Trial Center Functional Diagnostics Development Field researchers Izumi Umeda, who at the time was also a Project Researcher at the Kavli Institute for the Physics and Mathematics of the Universe (Kavli IPMU), and Hirofumi Fujii, alongside researchers from Chiba University, Kavli IPMU, and Kyoto College of Medical Science, combined components in nuclear medicine, Drug Delivery System (DDS) and complex chemistry, to develop a method that controls the in vivo dynamics of radionuclides post-administration.
The team’s method allows radiopharmaceuticals to accumulate sufficiently in cancer lesions, while at the same time, shortening the time drugs are in contact with healthy tissues. This means drugs can concentrate on the cancer lesions, improving its effects on the cancer lesions compared to the conventional methods.
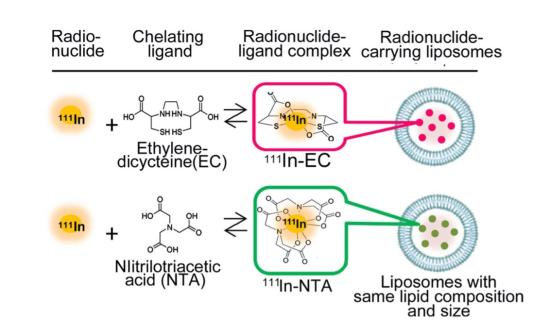
The two types of radiopharmaceuticals, or RI-encapsulated liposomes (Figure 1), have the same liposome composition and particle size, and the RI is the same 111In, differing only in the ligand. Nitrilotriacetic acid (NTA) is a typical ligand, and ethylenedicysteine (EC) is the new ligand at the center of the team’s experiment.
Two types of 111In-ligand-containing liposomes were given to mice with tumors, and researchers monitored the uptake and accumulation of 111In in cancer and healthy tissues, especially the liver and spleen (Figure 2). There was little difference in the tumors in both mice, but in the liver and spleen, 111In-EC-liposomes were once taken up but then rapidly cleared out, whereas conventional 111In-NTA-liposomes remained for a long time. The 111In-EC-liposomes also promoted excretion into feces, and the chemical form in urine was 111In-EC not in the liposomes.
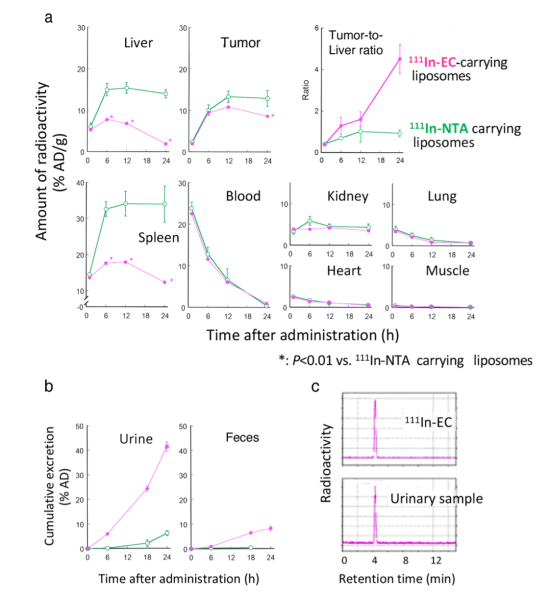
The team found that in the liver, the liposomes were broken up, releasing the 111In-ligand complexes inside, whereas in the tumor, liposomes persisted intact for a long time and the 111In-ligand complexes remained in liposomes. The unique complex, 111In-EC, allowed for rapid clearance from the liver after released from the liposomes.
In the in vivo SPECT/CT images of tumor-bearing mice after administration of 111In-EC- or 111In-NTA-liposomes (Figure 3), the distribution of the two liposomes in the body was almost identical to begin with. However, later, 111In-EC-liposomes appeared clearly in tumor sites owing to a negligible signal from the liver and spleen, whereas 111In-NTA-liposomes were unable to selectively image tumors due to their low clearance from the above organs. The images were consistent with the biodistribution studies described above.

Based on the results of this study, the team proposed a scheme explaining the in vivo fate of radionuclide-carrying liposomes (Figure 4). After intravenous administration, conventional 111In-NTA-liposomes and new 111In-EC-liposomes circulate in the bloodstream, and accumulate to a similar extent in tumors. Most liposomes remain intact within the tumor for a long time, and consequently, the encapsulated radionuclide-ligand complexes are retained within liposomes. Furthermore, liposomes are taken up by the liver, where they are rapidly degraded and release their encapsulated 111In-ligand complexes.
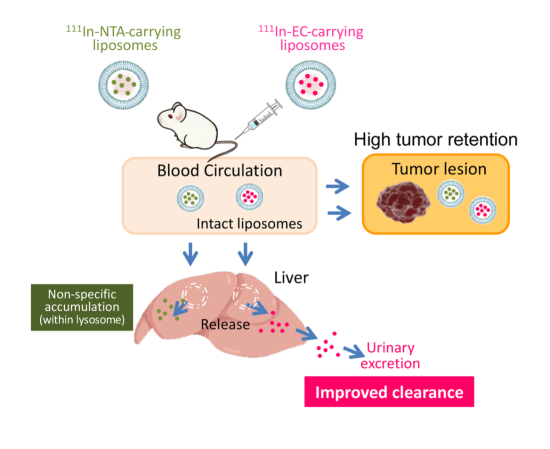
After their release from liposomes, conventional 111In-ligand complexes, such as 111In-NTA, remained in the liver for a long time. In contrast, 111In-EC were able to escape from the liver and was rapidly excreted in the urine in its original form. The researchers say rapid urinary excretion without accumulation in other tissues will allow radioactivity to be cleared swiftly from the body and minimize damage.
“The fact that a new approach to drug development has been developed at this time from a new perspective to maintain the concentration of drugs in cancer lesions while protecting healthy tissue is important when considering the role high-precision cancer treatment, or theranostics, will play in the treatment of the intractable. In the future, we hope that further research will lead to drugs that stay only in cancer lesions, which has never been done before,” said Umeda.
Details of their study were published in Journal of Controlled Release on August 23.
Scientific members and collaborators of this project
This research revolved around a joint project to develop novel radiopharmaceuticals for cancer diagnosis and therapy by National Cancer Center (NCC) and Chiba University. It incorporated the outcomes of a joint project by NCC and Kavli IPMU, aiming to apply space observation technology to medical applications, specifically in the development of high-precision gamma cameras, led by Kavli IPMU Professor Tadayuki Takahashi. Additionally, the study integrated the results of nuclear medicine data analysis research conducted in collaboration between the NCC and Kyoto College of Medical Science.
• Izumi Umeda (co-first and corresponding author), National Cancer Center Exploratory Oncology Research & Clinical Trial Center Functional Diagnostics Development Field researcher, and Project Researcher at the Kavli Institute for the Physics and Mathematics of the Universe at the time of research
• Yusuke Koike (co-first author), National Cancer Center, Graduate School and Faculty of Pharmaceutical Sciences, Chiba University
• Mayumi Ogata, National Cancer Center, Graduate School and Faculty of Pharmaceutical Sciences, Chiba University
• Emi Kaneko, Graduate School and Faculty of Pharmaceutical Sciences, Chiba University
• Shusei Hamamichi, National Cancer Center
• Tomoya Uehara, Graduate School and Faculty of Pharmaceutical Sciences, Chiba University
• Kunikazu Moribe, Graduate School and Faculty of Pharmaceutical Sciences, Chiba University
• Yasushi Arano, Graduate School and Faculty of Pharmaceutical Sciences, Chiba University
• Tadayuki Takahashi, Kavli Institute for the Physics and Mathematics of the Universe (WPI-Kavli IPMU), The University of Tokyo
• Hirofumi Fujii, National Cancer Center
Paper details
Journal: Journal of Controlled Release
Paper title: New liposome-radionuclide-chelate combination for tumor targeting and rapid healthy tissue clearance
Authors: Izumi O. Umeda (a, c, d), Yusuke Koike (a, b), Mayumi Ogata (a, b), Emi Kaneko (b), Shusei Hamamichi (a), Tomoya Uehara (b), Kunikazu Moribe (b), Yasushi Arano (b), Tadayuki Takahashi (c), Hirofumi Fujii (a)
Author affiliations:
a. Exploratory Oncology Research and Clinical Trial Center, National Cancer Center, 6-5-1, Kashiwanoha, Kashiwa, Chiba 277-8577, Japan
b. Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Cyuo-ku, Chiba, Chiba 260-8675, Japan
c. Kavli Institute for the Physics and Mathematics of the Universe (WPI), The University of Tokyo Institutes for Advanced Study, 5-1-5, Kashiwanoha, Kashiwa, Chiba 277-8583, Japan
d. Kyoto College of Medical Science, 1-3, Imakita, Oyama-higashi, Sonobe, Nantan, Kyoto 622-0041, Japan
DOI: doi.org/10.1016/j.jconrel.2023.07.060 (published 23 August 2023)
Paper (Journal of Controlled Release)
Enquiries
Motoko Kakubayashi
Press officer
Kavli Institute for the Physics and Mathematics of the Universe (Kavli IPMU)
The University of Tokyo
E-mail:press_at_ipmu.jp
* Please change _at_ to @






